👩⚕️ IBM Watson for Clinical Trial Matching
Watson for Clinical Trial Matching
Watson for Clinical Trial Matching (CTM) is a cloud-based, cognitive offering and essential resource for clinical research teams that promotes new cure and treatment discoveries while alleviating administrative burdens associated with identifying and managing patients for clinical trials. With the intelligence of Watson, patients can now be pre-screened in seconds versus the current time-consuming process involving coordinator chart reviews for every patient. CTM greatly reduces the time and effort involved with identifying patients and increases the number of patients who will be offered trials as a treatment option.
The result is better trials, better treatment options, faster accrual, more medical knowledge applied to clinical practice, more patients on trials, improving lives, and giving hope through data and cognitive insights.
Clinical trials allow for the testing of new treatments that have the potential to improve patient conditions and health. Recruiting for trials is a daunting, long process that can take years. Many trials do not meet enrollment requirements and this leaves potentially life saving treatments falling short of regulatory approval and approximately 29% of a clinical trial's costs can be attributed to the enrollment process.
In 2015, IBM Watson Health collaborated with the Mayo Clinic to develop Watson for Clinical Trial Matching (CTM) to help identify patients for clinical trials. This was done using the Watson API for matching trial criteria with patient conditions.
My Role
I joined Watson Health in 2016 to reimagine the clinical trial enrollment and screening experience. I was the UX Lead responsible for the overall design approach aligning with the Director of Design, Offering Managers and Project Architects. I was responsible for planning and facilitating design thinking workshops around the country including at the Mayo Clinic, conducting daily team standups, designing and presenting findings to executive leadership, and working alongside a team of brilliant designers, engineers, and researchers. Together, we developed the interactions, prototyped low and high fidelity concepts, tested concepts with trial coordinators and research managers, and delivered a product that helped the Mayo Clinic experience an 80% increase in Breast Cancer trial enrollment. This is most definitely one of my career highlights and being a part of this team was an honor.
Target User Challenges
Clinical Trial Coordinators are responsible for patient recruitment and enrollment, but Oncologists were the original target audience for the product. This presented many challenges, and our team conducted interviews and workshops with medical professionals at the Mayo Clinic and MD Anderson Cancer Center. Our research resulted in a product shift. What was once patient and oncologist-centered became coordinator and research manager-centered. This was a huge challenge, as our work needed to focus on all of the tasks coordinators and managers conduct.
Binders Everywhere

Clinical Trial Coordinators come from a variety of backgrounds, but many have nursing or other medical experience. They are responsible for finding patients by attending meetings and tumor boards, working with physicians, reviewing charts, tracking patients from recruitment to enrollment, managing the patients on the trial, and collecting data from the trials for audits. This is a messy process and it's common for this to be managed with post-its, emails, spreadsheets, and hard copies of documents. It's common to see coordinators working in densely packed offices full of binders and paper.
The Guinea Pig Stigma
When interviewing a potential patient for a trial, there is often fear. Not only are patients less likely to enroll in a trial for experimental drugs, they are also less likely to do so if pre-approved drugs already exist for a condition. On top of this, the most up-to-date patient information is needed for physicians and care providers to identify potential trials and this is not something that can be identified instantly. By allowing Watson to connect these dots, it could help provide patients faster access to potentially life-saving trials and treatments they would not normally learn about without a fast, current, and automated identification system. Reducing the time to trial enrollent by eliminating the human-to-human communication that slows down the current enrollment process is a win-win for patients and trial coordinators. Imagine a system that could identify you for a trial based on your most current condition, procedures, lab tests, blood samples, and biopsies in a fraction of the time it would take a human to do this. What once could take years, could now be done in minutes, and possibly seconds. This isn't hyperbole!
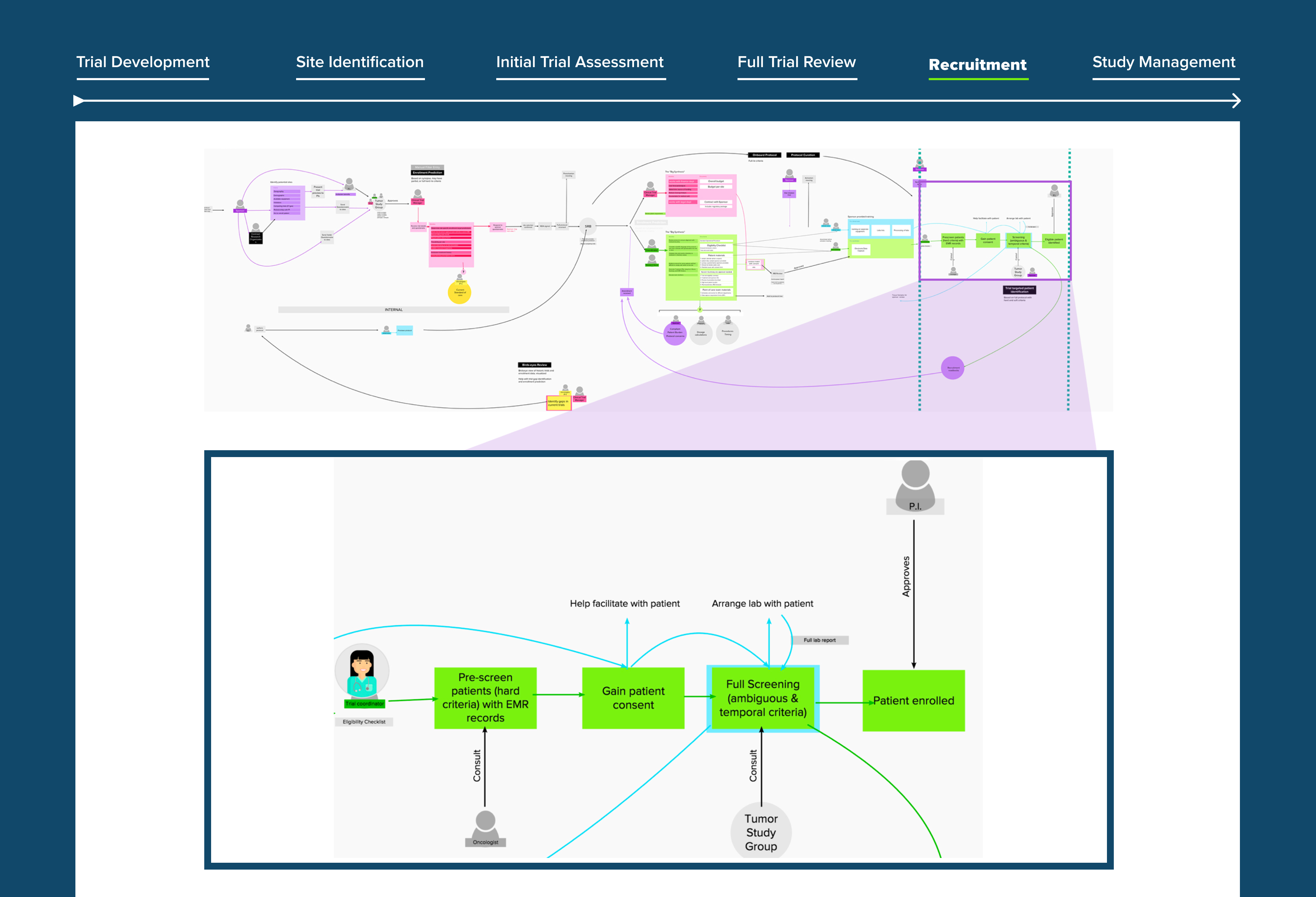
👆 Our team worked endlessly on understanding the workflow of our new target users.
"It starts with my trials."
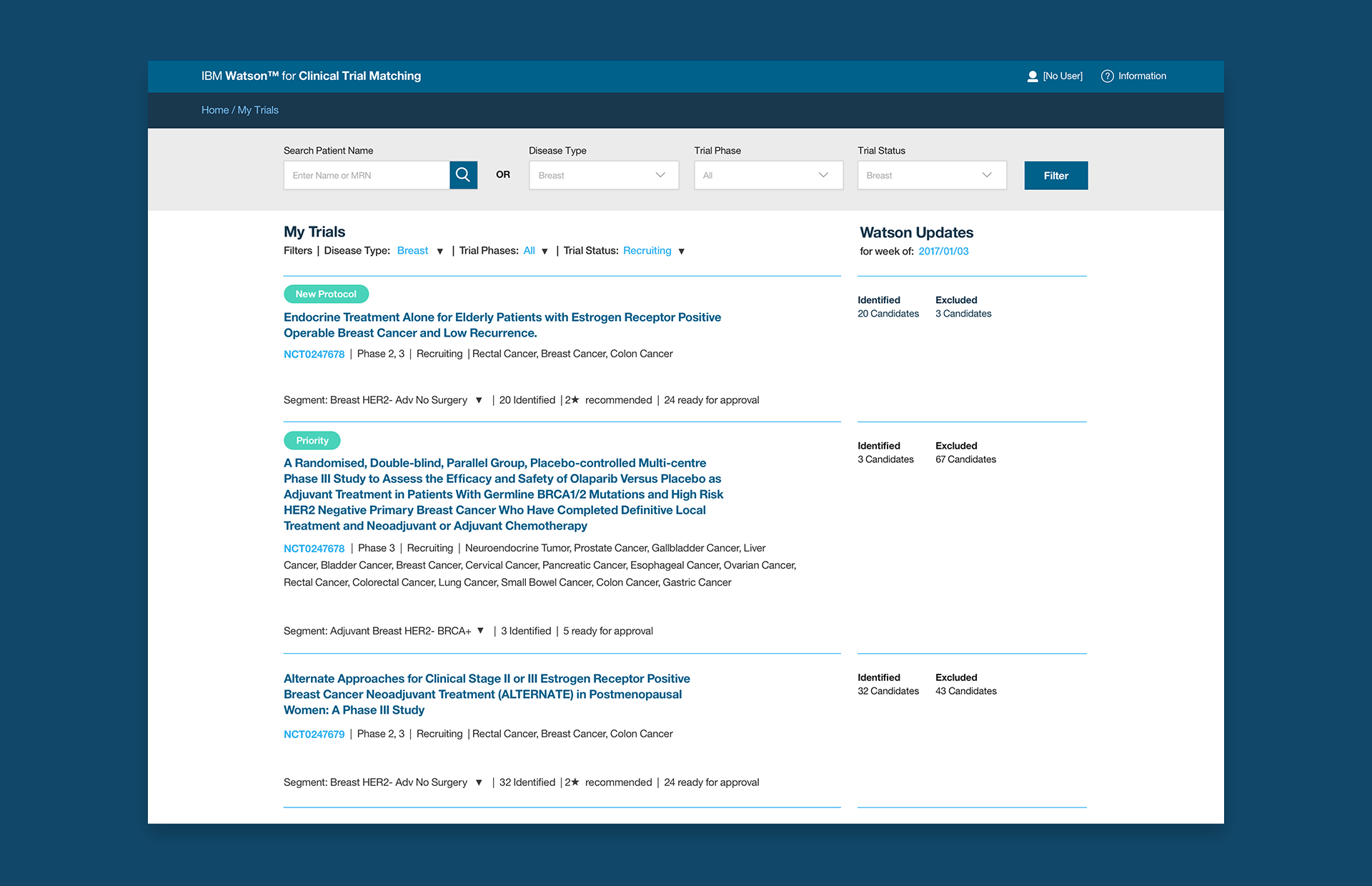
We landed on a list of trials that each coordinator was working on. We called it My Trials. Taken directly from a coordinator interview, it seemed to make perfect sense. We started with what matters most to them.
From My Trials, our workflow would provide a few avenues into and out of patient and trial information. This overview would provide coordinators and research managers with a full project management overview of their entire patient list and where they were in the enrollment process. This was an actionable list of items that would assist users with the important decisions needed to move a patient forward and into the enrollment flow of Tracker.
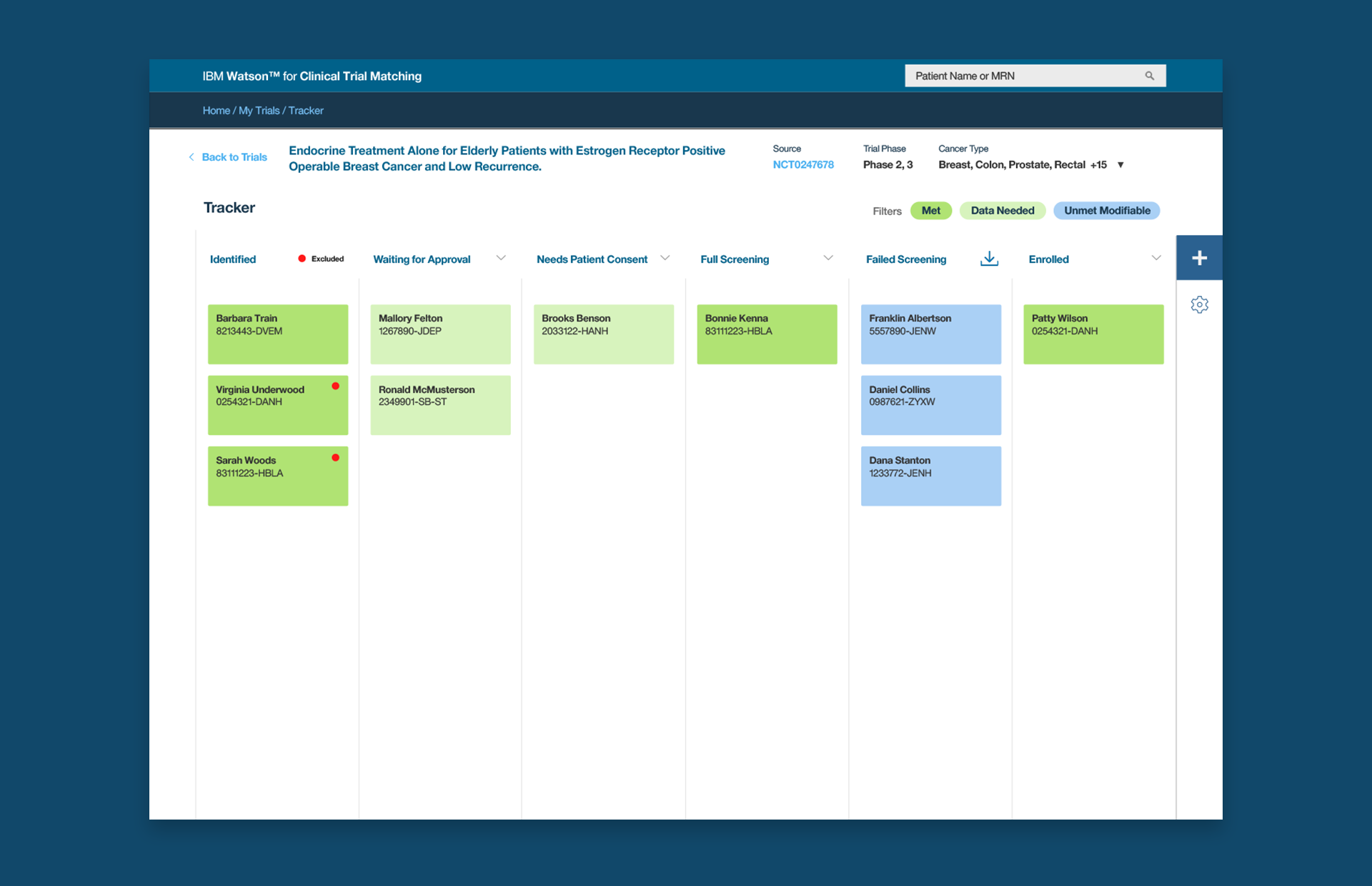
Tracker - The Future of Clinical Trials
👆Watson at Work: Identifying patients for potential enrollment and flagging candidates with actionable color coding
Tracker is one of my top career accomplishments. I am so proud of our work on this feature. It was created after an experience at a coordinator's office, where a wall of sticky notes related to a trial covered the wall. Essentially, Tracker is a kanban board for clinical trials. Tracker allowed us to capture the identified-to-enrolled flow of a clinical trial, while utilizing the intelligence of Watson to automate the lists. When Watson spots a potential candidate, they are added to Tracker with actionable color coding, which was familiar to trial coordinators and managers using colored post-it notes on their office walls.
This would lead them to next steps, where they could change status manually. This was an important human intervention in the process, as there was some fear of having this workflow completely automated and out of the hands of experienced medical professionals. There is a line where artificial intelligence and machine learning needs to earn trust and this was a great place to make use of the technology while reducing the fear and risk of error. They loved it. It was never intended to replace humans. It was intended to enhance their skills, free their time, and reduce the labor involved with lengthy and exhausting recruitment.
Ideas, Wireframes, and Other Notable Screens
👆 Prototype: Tracker accessibility controls via keyboard navigation (Try it out!)
In early explorations of Tracker, we concepted various methods for color coding different patient statuses. Coordinators did this manually using post-its, spreadsheets, and other methods.
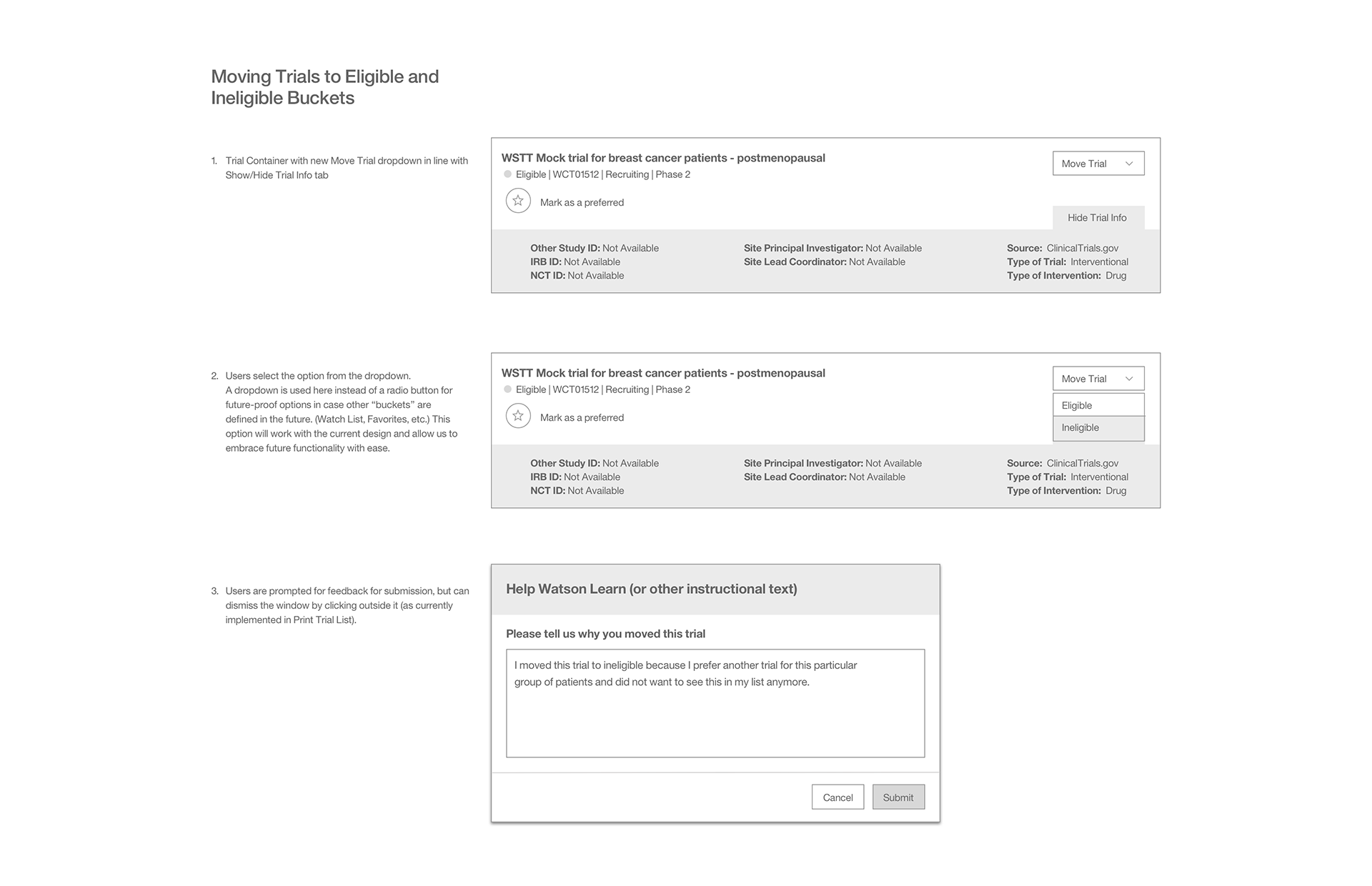
As a part of our work, we needed to explore how to move trials to eligible and ineligible containers.
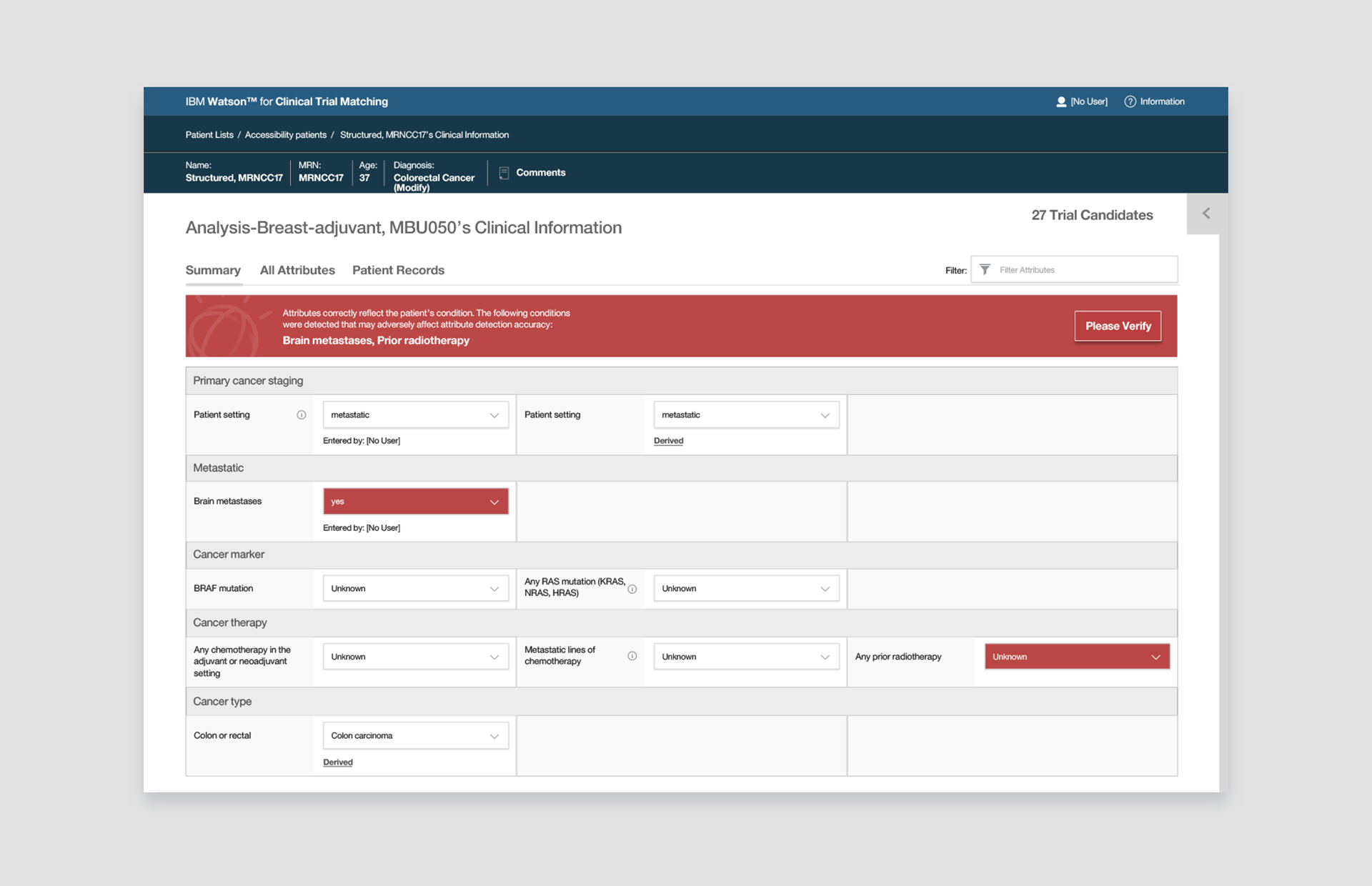
👆 NLP Alerts: When Watson needs attention
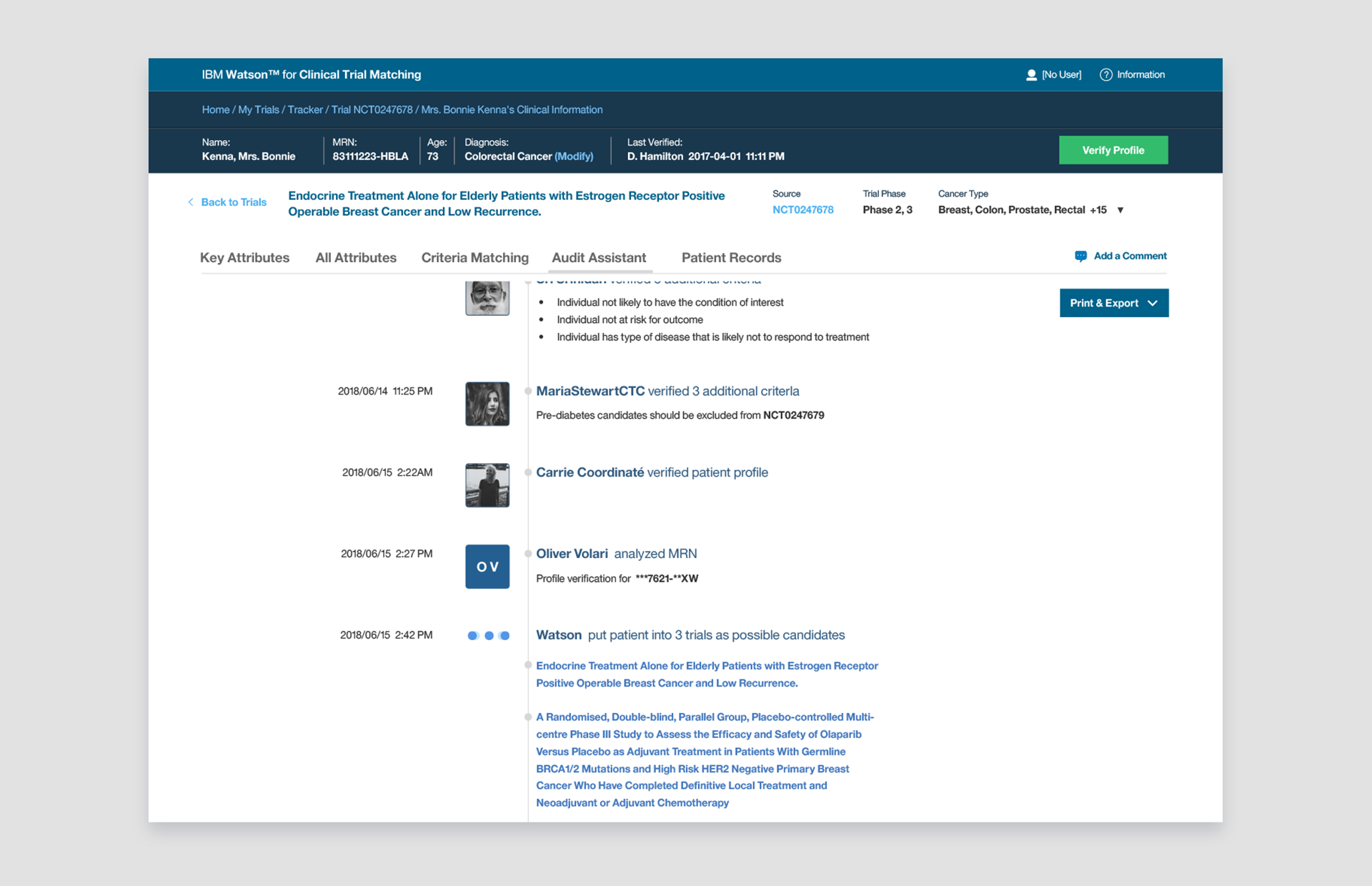
👆 Audit Assistant: When teams need to communicate